- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
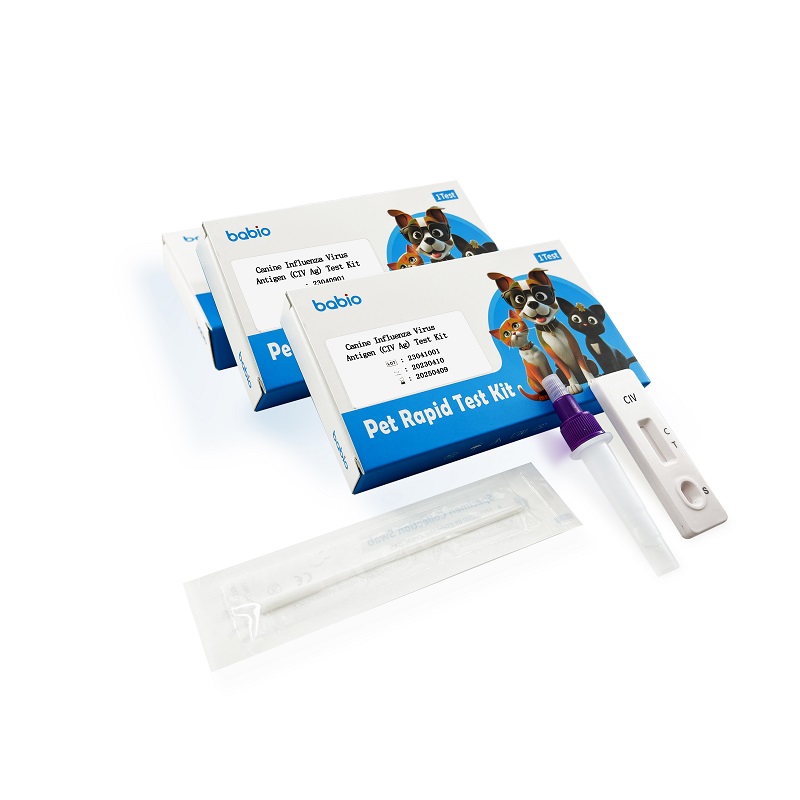
Canine Influenza Virus Antigen (CIV Ag) Test Kit
Canine Influenza Virus Antigen (CIV Ag) Test Kit rapidly detects the canine influenza virus antigen in canine nasal secretions, and is used for canine influenza virus infection screening and auxiliary diagnosis.
Send Inquiry
Canine Influenza Virus Antigen (CIV Ag) Test Kit
Canine Influenza Virus Antigen (CIV Ag) Test Kit rapidly detects the canine influenza virus antigen in canine nasal secretions, and is used for canine influenza virus infection screening and auxiliary diagnosis.
【testing principle】
Canine influenza viruses are emerging pathogens that can cause severe acute respiratory disease in dogs, which may be mild or severe. The vast majority of dogs are not clinically obvious. The common clinical manifestation is cough, which usually lasts from 10 to 30 days, although antibiotic treatment has relieved the cough. Most dogs show wet cough, others show dry cough similar to kennel cough. Most dogs have purulent discharge and low-grade fever. Some dogs present with a more severe infection and develop pneumonia along with high fever and breathing difficulties.
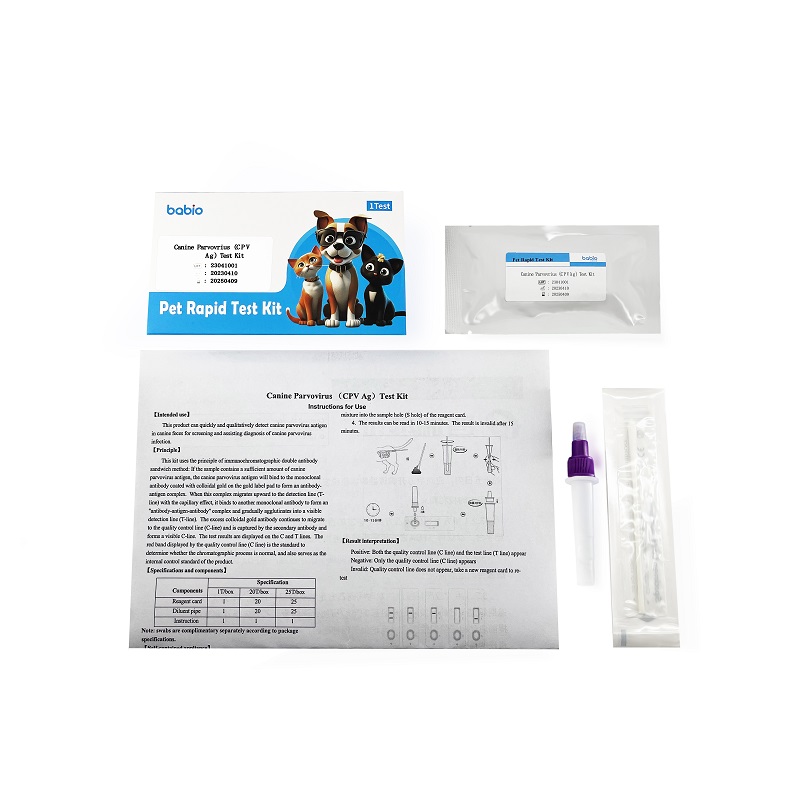
This kit was performed by double-antibody sandwich immunochromatography. If the sample contains sufficient amounts of the canine influenza virus antigen, the canine influenza virus antigen binds to a monoclonal antibody coated with the gold standard pad to form an antibody-antigen complex. The complex with the capillary effect upward migration to the detection line (T line), combined with another monoclonal antibody to form "antibody-antigen-antibody" complex and gradually agglutinate into a visible detection line (T line), excessive colloidal gold antibody continue to migrate to the quality control line (C line) is captured by secondary antibody and form visible C line. Test results are shown by the C and T lines. The red band shown by the quality control line (the line C) is the standard to determine whether the chromatography process is normal, and also serves as the internal control standard of the product.
【 Package Specifications and components 】
| Components | Specification | ||
| 1T/box | 20T/box | 25T/box | |
| Reagent card | 1 | 20 | 25 |
| Diluent pipe | 1 | 20 | 25 |
| Instruction | 1 | 1 | 1 |
Note: according to the packaging specifications, other equipment can be purchased or contact the manufacturer.
【Self-contained appliance】
Timepiece
【Storage and expiration date】
The kit is stored at 2-30℃. Do not freeze. Valid for 24 months; After the kit is opened, the reagent should be used as soon as possible.
【Sample requirement】
1. Sample:dog nasal secretions.
2. Samples should be tested on the same day; Samples that cannot be tested on the same day should be stored at 2-8 ° C, and those that exceed 24 hours should be stored at -20 ° C.
【Specimen collection and processing】
Nasal: insert a sterile cotton swab into the nostrils, parallel to the nostrils and place for a few seconds to absorb secretions. Samples were collected using nasal swabs for optimal performance.
The above swab with the collected sample was inserted into the dilution tube and fully mixed with the liquid. Finally, the swab was squeezed to leave most of the solution in the extraction tube, then the swab was removed and the cap was tightened. Optimal results are obtained if the samples are tested immediately after collection.
【Inspection method】
1. Before use, restore the kit to room temperature (15-30℃).
2. Remove the reagent card from the aluminum foil bag and place it on a clean platform.
3. Unscrew the top tube cap on the diluent tube cap containing the sample, invert the diluent tube, squeeze the tube wall, and add 3-5 drops of specimen mixture into the sample hole (S hole) of the reagent card.
4. The results can be read in 10-15 minutes. The result is invalid after 15 minutes.
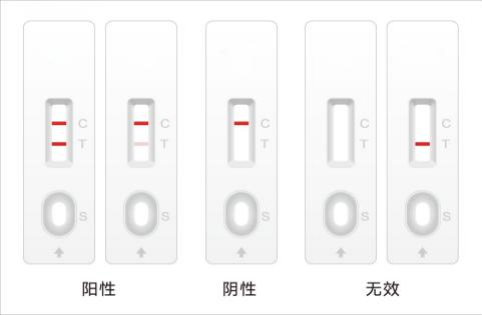
【 Result interpretation 】
Positive: Both the quality control line (C line) and the test line (T line) appear
Negative: Only the quality control line (C line) is available
Invalid: Quality control line does not appear, take a new device to retest
【Precautions】
1. This product is only used for qualitative testing and does not indicate the virus level in the sample.
2. Test results of this product are for reference only and should not be used as the sole basis for diagnosis and treatment, but should be made by a physician after evaluating all clinical and laboratory evidence.
3. A negative result may occur if the viral antigen present in the sample is below the detection limit of the assay, or if the antigen detected at the disease stage at which the sample was collected is not present.
4. The operation should be carried out in strict accordance with the instructions. Do not use expired or damaged products.
5. The test card should be used within 1 hour after opening; If the ambient temperature is higher than 30 ° C or more humid, it should be used immediately.
6. If the T line has just begun to show color, and then the line color gradually fades or even disappears, in this case, the sample should be diluted several times and tested until the T line color is stable.
7. This product is a disposable product. Do not reuse it.