- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Babio's novel Coronavirus Antigen products won the "white List" of many countries
2021-09-17
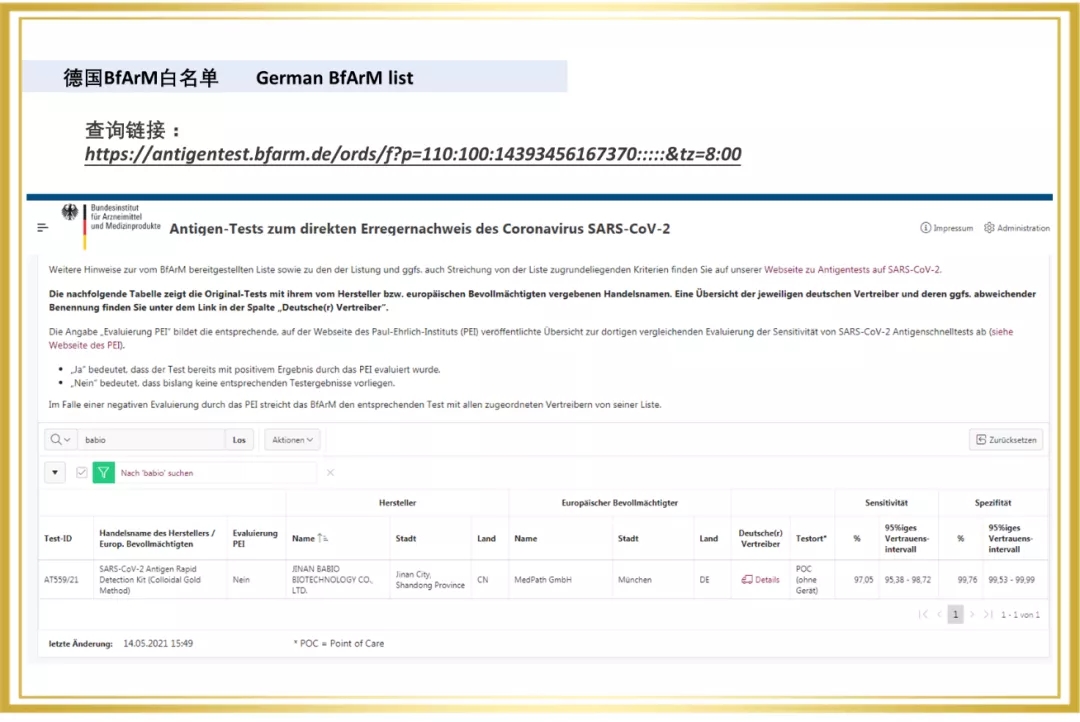
Recently, The Novel Coronavirus (2019-NCOV) antigen test (colloidal gold method) independently developed by Babco has won the "whitelist" of Belgium and Portugal, adding a new tool to the global fight against COVID-19.
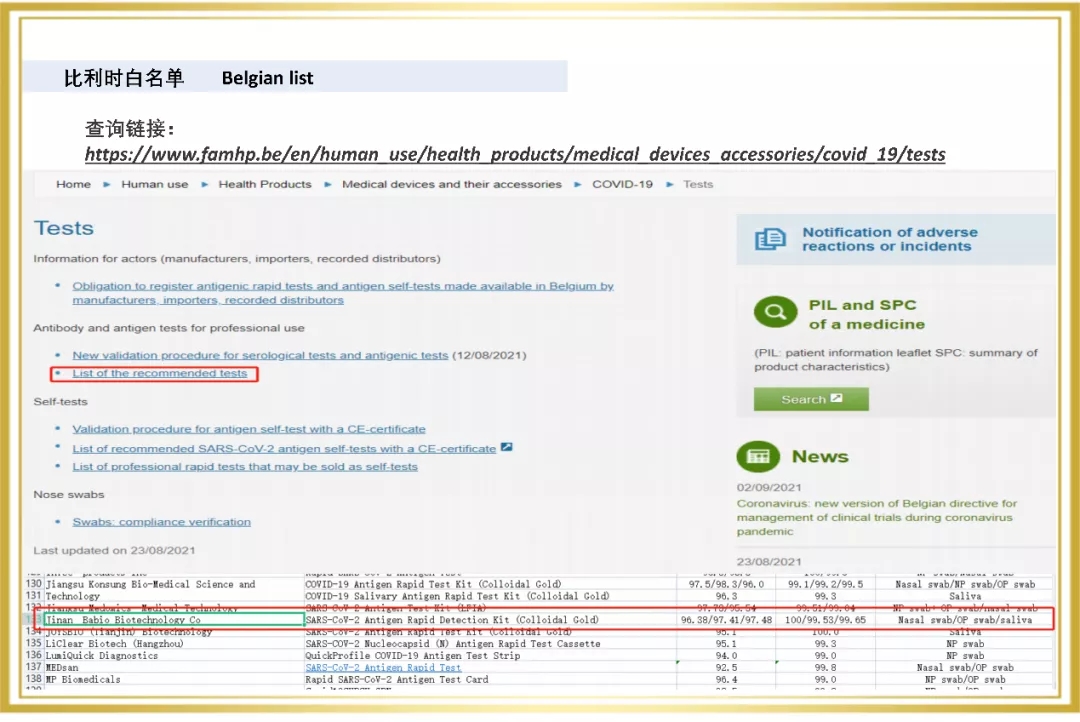

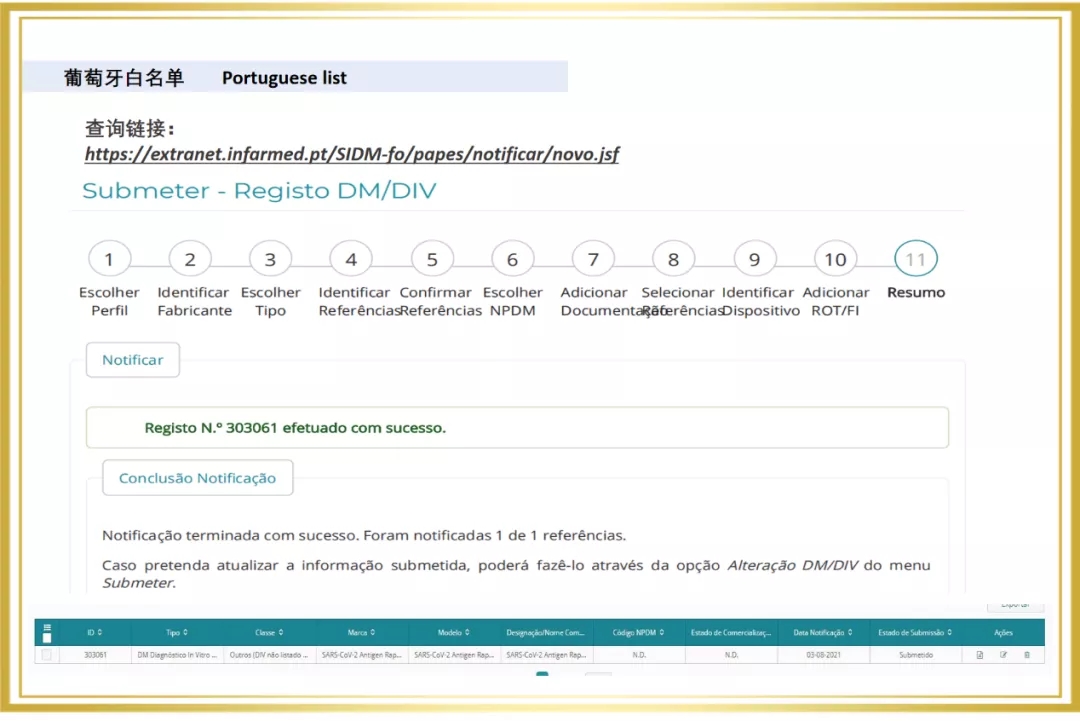
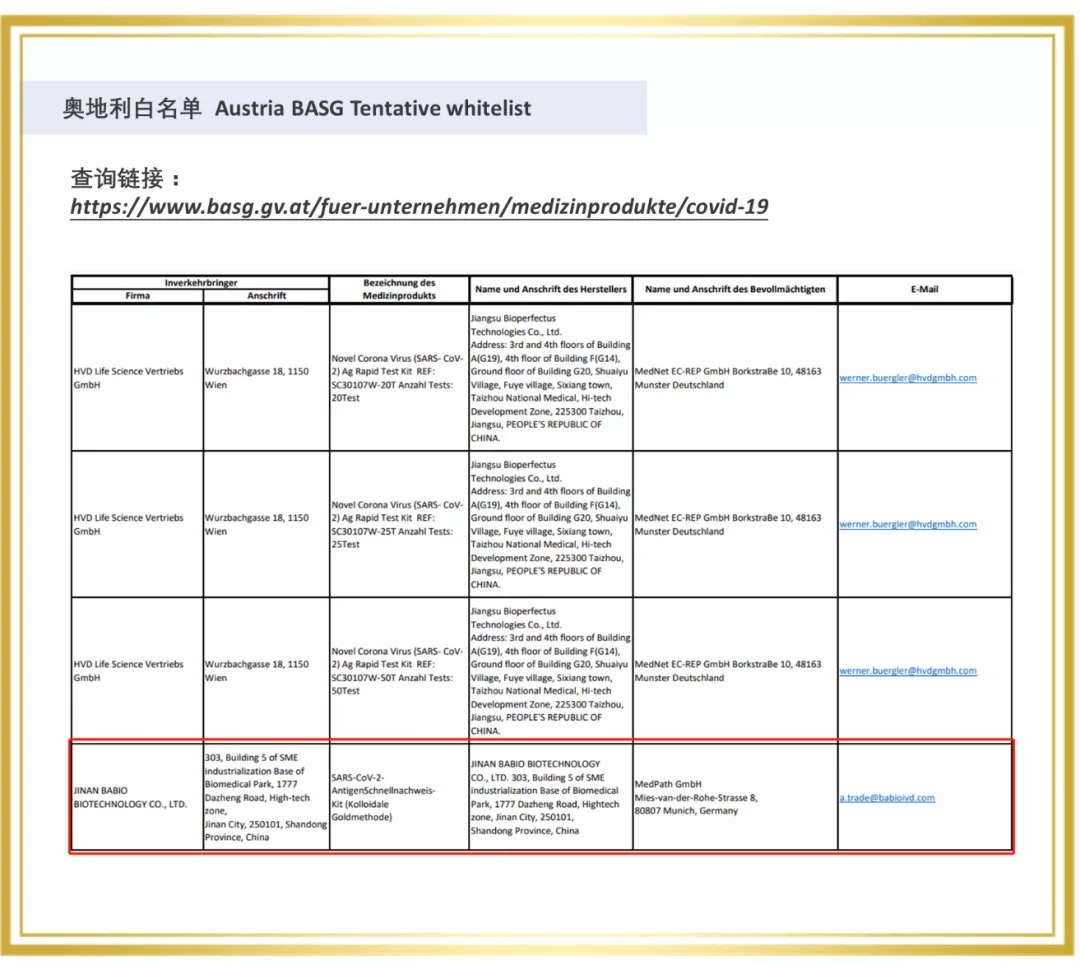
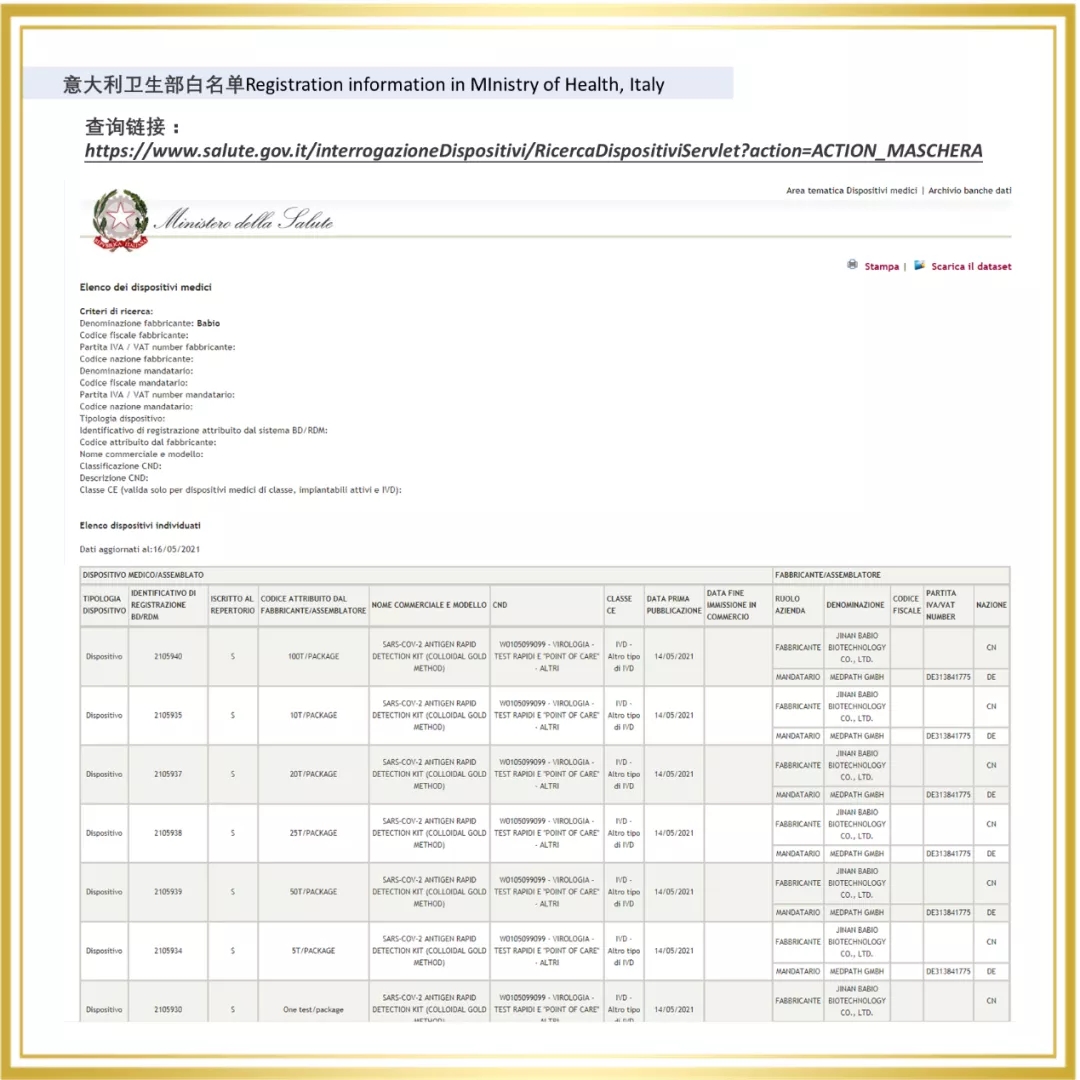
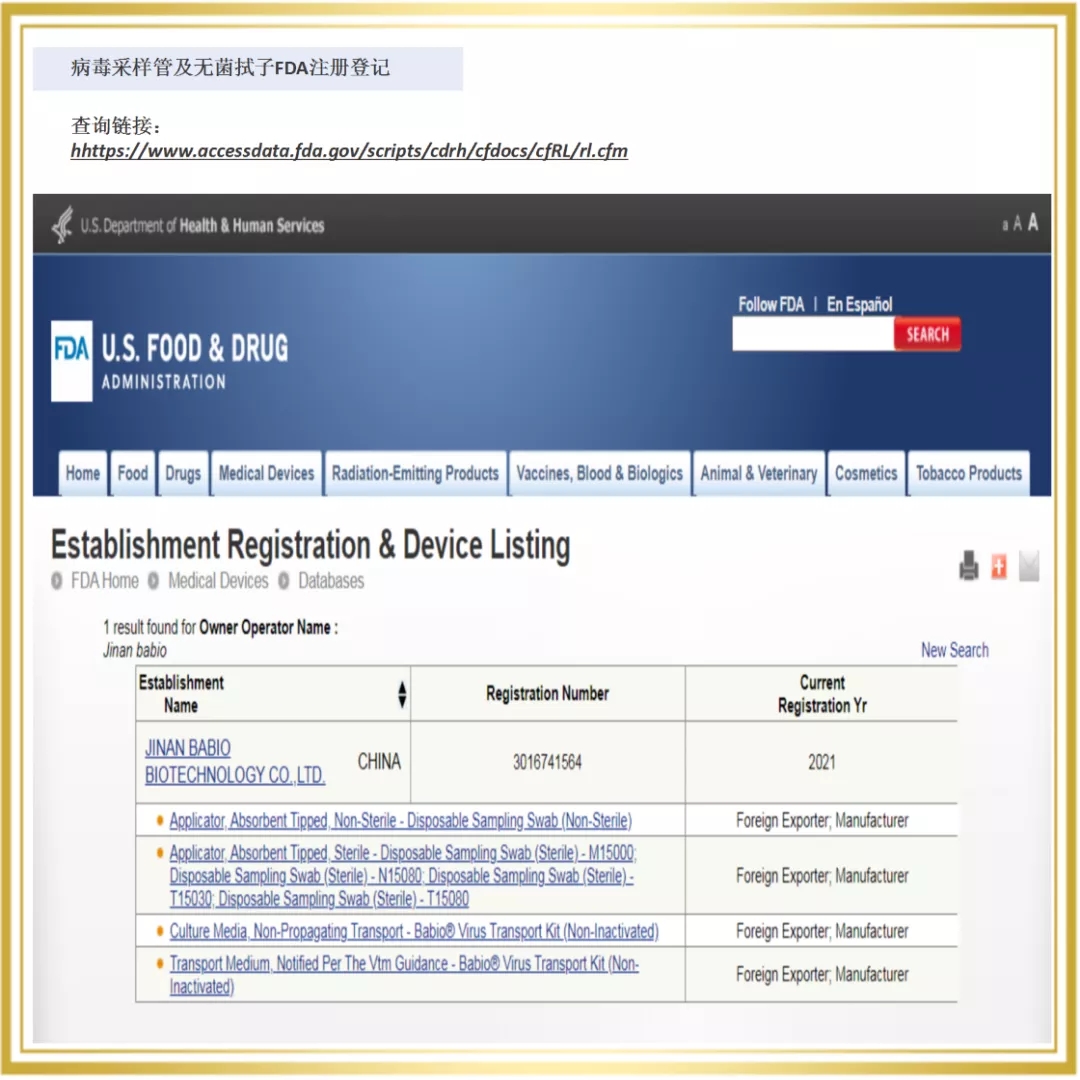
Since 2020, Babio Bio has entered the overseas market, and a number of rapid detection products are rapidly realizing the global layout. In 2021, The Company passed the certification of a number of European and American authorities, for example, Novel Coronavirus (SARS-COV-2) Rapid antigen detection reagent (colloidal gold method) obtained the "White List" of Germany BfArM, Italy Ministry of Health, Austria, etc. At the same time, Babio biological virus sampling tube on the EUA white list of the United States FDA, the enterprise through ISO13485 certification, sterile swab issued by Intertek CE certificate (announcement agency no. : 0413) and FDA.