- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
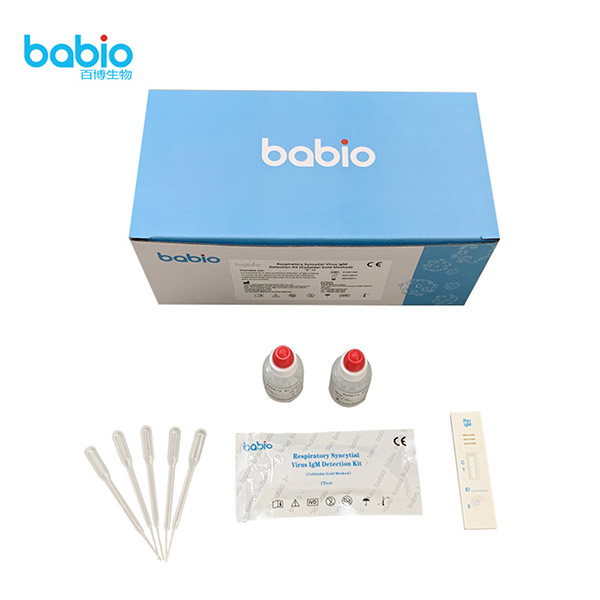
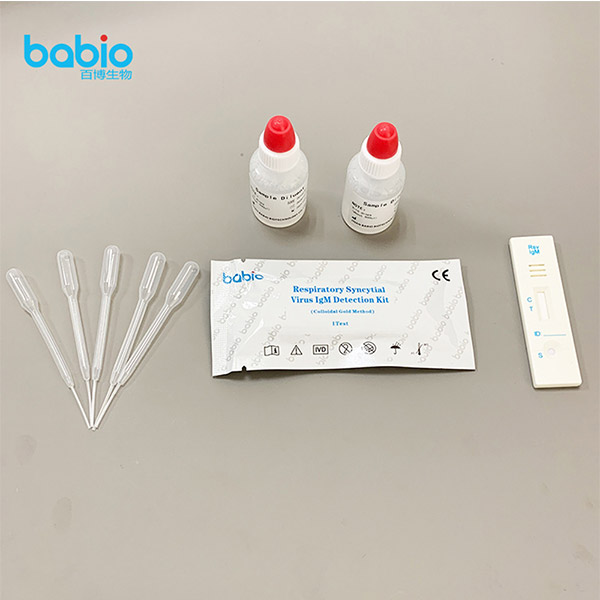
Respiratory Syncytial Virus IgM Detection Kit (Colloidal Gold Method)
As a professional Respiratory Syncytial Virus IgM Detection Kit (Colloidal Gold Method) manufacture, you can rest assured to buy Respiratory Syncytial Virus IgM Detection Kit (Colloidal Gold Method) from our factory and we will offer you the best after-sale service and timely delivery.
Send Inquiry
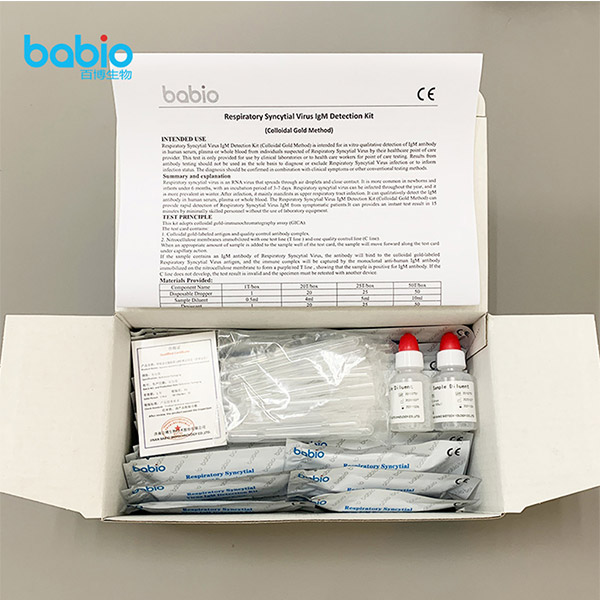
INTENDED USE
The Respiratory Syncytial Virus IgM Detection Kit (Colloidal Gold Method) is intended for in vitro qualitative detection of IgM antibody in human serum, plasma or whole blood from individuals suspected of Respiratory Syncytial Virus by their healthcare point of care provider. This test is only provided for use by clinical laboratories or to health care workers for point of care testing. Results from antibody testing should not be used as the sole basis to diagnose or exclude Respiratory Syncytial Virus infection or to inform infection status. The diagnosis should be confirmed in combination with clinical symptoms or other conventional testing methods.
Summary and explanation
Respiratory syncytial virus is an RNA virus that spreads through air droplets and close contact. It is more common in newborns and infants under 6 months, with an incubation period of 3-7 days. Respiratory syncytial virus can be infected throughout the year, and it is more prevalent in winter.
After infection, it mainly manifests as upper respiratory tract infection. It can qualitatively detect the IgM antibody in human serum, plasma or whole blood. The Respiratory Syncytial Virus IgM Detection Kit (Colloidal Gold Method) can provide rapid detection of Respiratory Syncytial Virus IgM from symptomatic patients.It can provides an instant test result in 15 minutes by minimally skilled personnel without the use of laboratory equipment.
TEST PRINCIPLE
This kit adopts colloidal gold-immunochromatography assay (GICA).
The test card contains:
1. Colloidal gold-labeled antigen and quality control antibody complex.
2. Nitrocellulose membranes immobilized with one test line (T line ) and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an IgM antibody of Respiratory Syncytial Virus, the antibody will bind to the colloidal gold-labeled Respiratory Syncytial Virus antigen, and the immune complex will be captured by the monoclonal anti-human IgM antibody immobilized on the nitrocellulose membrane to form a purple/red T line , showing that the sample is positive for IgM antibody. If the C line does not develop, the test result is invalid and the specimen must be retested with another device.
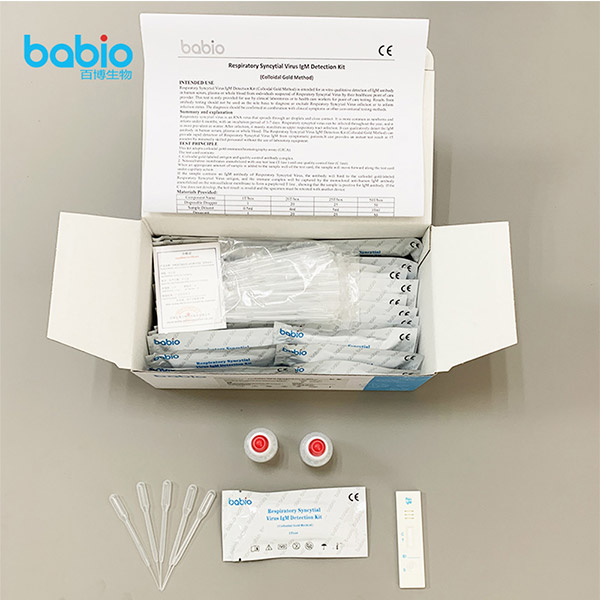
Materials Provided

TEST PROCEDURE
Step1: Allow the test device, buffer, specimen to equilibrate to room temperature (15-30℃) prior to testing.
Step2: Remove the test device from the sealed pouch. Place the test device on a clean, flat surface.
Step3: Label the device with specimen number.
Step4: Using a Disposable Dropper, transfer serum, plasma or whole blood. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μ l) to the specimen well(S) of the test device, and immediately add 2 drops of test buffer (approximately 70-100μ l). Make sure there are no air bubbles.
Step5: Set up a timer. Read the results in 15 minutes.
Do not interpret the result after 20 minutes.To avoid confusion, discard the test device after interpreting the result. If you need to store it for a long time, please take a photo of the result.

1.NEGATIVE RESULT:
If only the C line develops, the test indicates that no detectable antibody is present in the specimen. The result is negative or non-reactive.
2. POSITIVE RESULT:
In addition to the presence of the C line, if the T line develops, the test indicates the presence of IgM antibody. The result is positive or reactive.
3. INVALID
If the C line does not develop, the assay is invalid regardless of color development of the T line as indicated below. Repeat the assay with a new device.