- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Home
>
Products > Human Rapid Test Kit > Tropical Disease Rapid Test > Malaria P.f/P.v Antigen Combination Test kit (Whole blood)
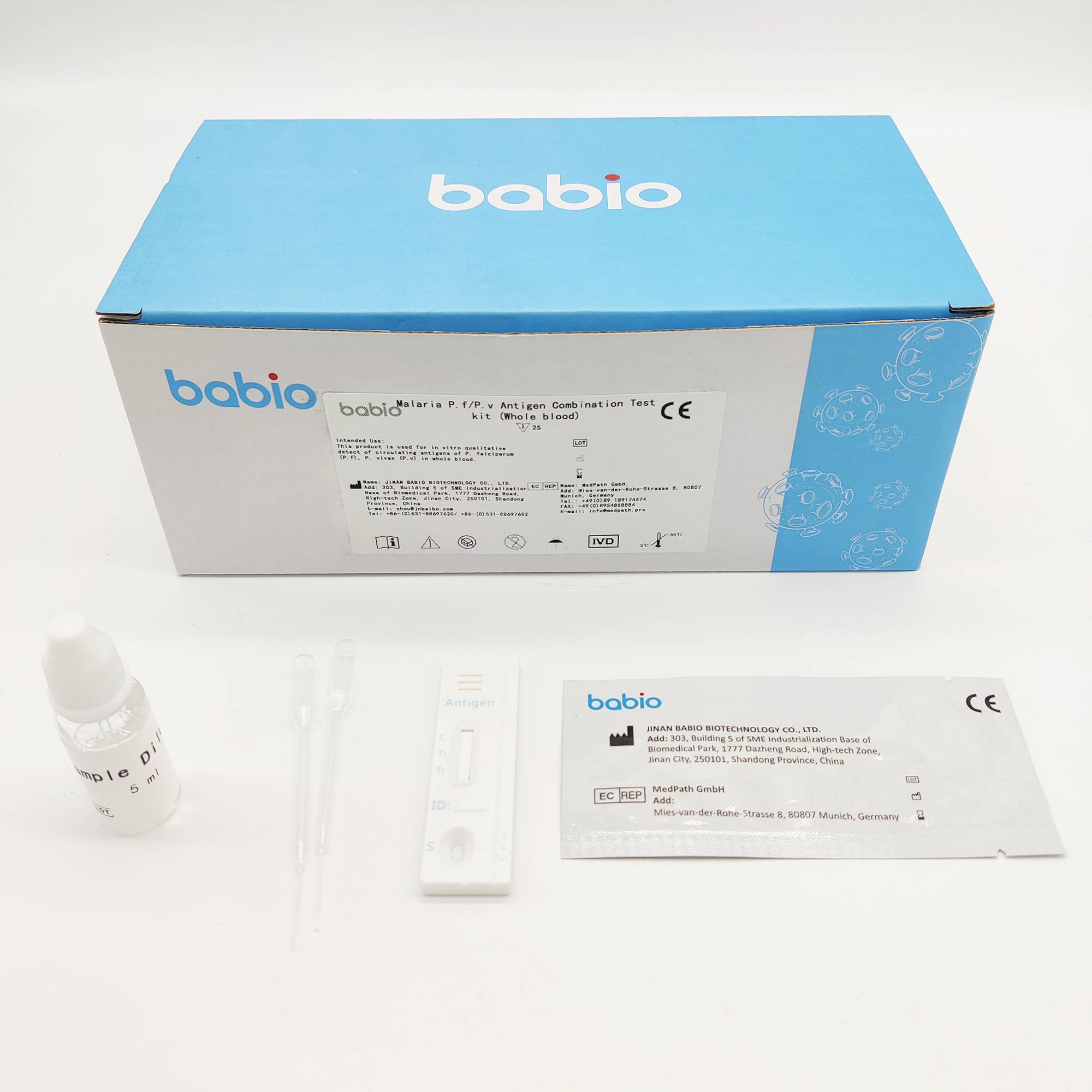
Malaria P.f/P.v Antigen Combination Test kit (Whole blood)
Malaria P.f/P.v Antigen Combination Test kit (Whole blood) is used for in vitro qualitative detect of circulating antigens of P. falciparum (P.f), P. vivax (P.v) in whole blood.
Send Inquiry
Product Description
Product Description
INTENDED USE
This product is used for in vitro qualitative detect of circulating antigens of P. falciparum (P.f), P. vivax (P.v) in whole blood.
Summary and explanation
Malaria is caused by a protozoan which invades human red blood cells. Malaria is one of the world's most prevalent diseases. According to the WHO, the worldwide prevalence of the disease is estimated to be 300-500 million cases and over 1 million deaths each year. Most of these victims are infants, young children. Over half of the world's population lives in malarious areas. Microscopic analysis of appropriately stained thick and thin blood smears has been the standard diagnostic technique for identifying malaria infections for more than a century. The technique is capable of accurate and reliable diagnosis when performed by skilled microscopists using defined protocols. The skill of the microscopist and use of proven and defined procedures, frequently present the greatest obstacles to fully achieving the potential accuracy of microscopic diagnosis. Although there is a logistical burden associated with performing a time-intensive, labor-intensive, and equipment-intensive procedure such as diagnostic microscopy, it is the training required to establish and sustain competent performance of microscopy that poses the greatest difficulty in employing this diagnostic technology.
Malaria P.f/P.v Antigen Combination Test kit (Whole blood) is an immunological diagnostic test used for detection of circulating antigens of P. falciparum (P.f), P. vivax (P.v) based on the colloidal gold-immunochromatography assay. This method is rapid and convenient to use and requires few equipment.It can be performed within 15-20 minutes by minimally skilled personnel.
TEST PROCEDURE
1. Allow the test device,diluent, specimen to equilibrate to room temperature (15-30℃) prior to testing.
2.Remove the test device from the sealed pouch. Place the test device on a clean, flat surface.
3.Label the device with specimen number.
4.Using a Disposable Dropper, transfer whole blood. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10-30μl) to the specimen well(S) of the test device, and immediately add 2 drops of diluent (approximately 70-100μl). Make sure there are no air bubbles.
5.Set up a timer. Read the results in 15 minutes.
Do not interpret the result after 20 minutes. To avoid confusion, discard the test device after interpreting the result. If you need to store it for a long time, please take a photo of the result.
Materials Provided
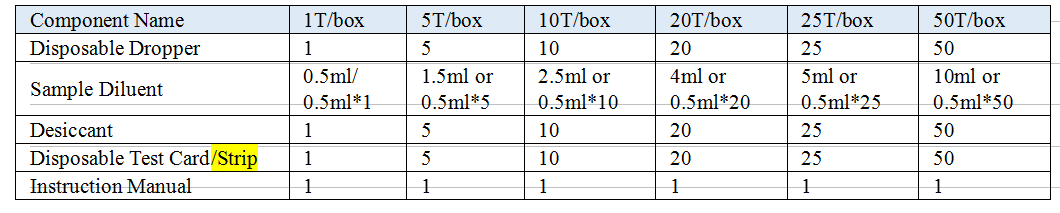
Model: Test Card, Test Strip
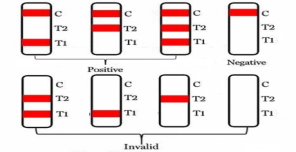
RESULTS
Positive:A red line appears at the position of the quality control line (C line) and the detection line (T1 line), which indicates the test result of circulating antigens of P. falciparum (P.f) in the sample was positive. A red line appears at the position of the quality control line (C line) and the detection line (T2 line), which indicates the test result of circulating antigens of P. vivax (P.v.) in the sample was positive. A red line appears at the position of the quality control line (C line) and the detection lines (T1 line and T2 line), which indicates the test result of circulating antigens of P. falciparum (P.f), P. vivax (P.v.) in the sample was positive.
Negative: If only the C band is present, indicates that no circulating antigens of P. falciparum (P.f), P. vivax (P.v) is detected in the specimen. The result is negative.
Invalid: The control line fails to appear.Review the procedure and repeat the procedure with a new kit. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Hot Tags: Malaria P.f/P.v Antigen Combination Test kit (Whole blood), Manufacturers, Suppliers, Wholesale, Buy, Factory, Customized, In Stock, Bulk, Free Sample, Brands, China, Made in China, Cheap, Discount, Low Price, CE, Fashion, Newest, Quality, Advanced, Durable, Easy-maintainable
Product Tag
Related Category
Drug Abuse Test
Covid-19 and Monkeypox Rapid Test
Hepatitis Virus Rapid Test
Tropical Disease Rapid Test
Respiratory Rapid Test
Cardiac Marker Test Kit
Tumor Marker Test Kit
Other Rapid Test kits
Send Inquiry
Please feel free to give your inquiry in the form below. We will reply you in 24 hours.