- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
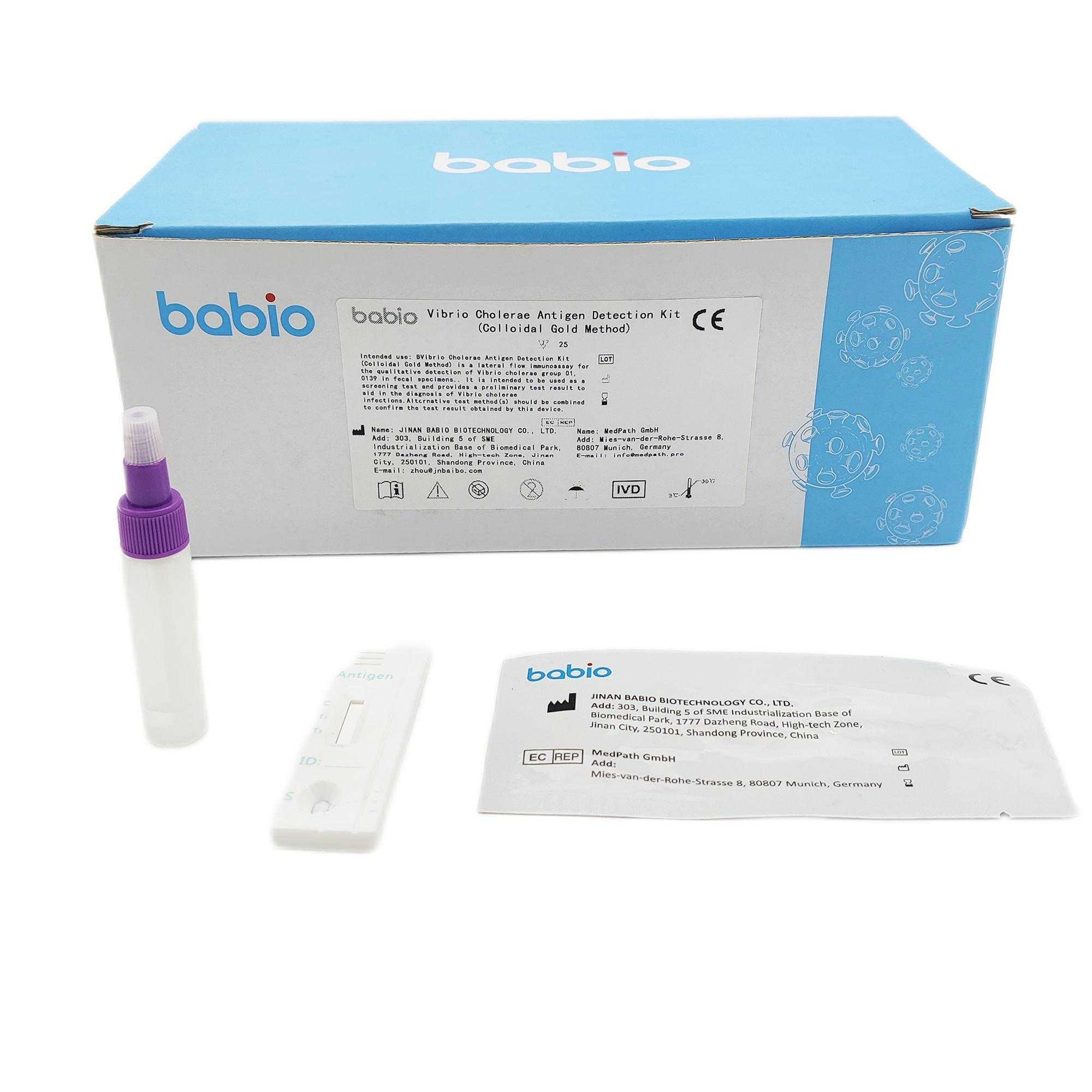
vibrio cholera rapid diagnostic test Manufacturers
Our organization puts emphasis to the administration, the introduction of talented personnel, and also the construction of team building, making an attempt hard to enhance the standard and liability consciousness of workers members. Our business successfully attained IS9001 Certification and European CE Certification of vibrio cholera rapid diagnostic test,vibrio cholera rapid test kit,vibrio cholera rapid diagnostic test kit,vibrio cholerae express test,vibrio cholera rapid test kit, Initial enterprise, we understand each other. Additional enterprise, the trust is getting there. Our firm usually at your service anytime.
vibrio cholera rapid diagnostic test, Our company has a skillful sales team, strong economic foundation, great technical force, advanced equipment, complete testing means, and excellent after-sales services. Our goods have beautiful appearance, fine workmanship and superior quality and win the unanimous approvals of the customers all over the world.
Hot Products
Brucella Canis Antibody (B. canis Ab) Test Kit
Brucella Canis Antibody (B. canis Ab) Test Kit is based on fast immuno-chromatography technique to detect Brucella Ab in sheep and bovine blood and milk. It is convenient, fast, sensitive and can be used for on-site large-scale testing. C line appears, means the test is valid. T line appears, means there is Brucella Ab in the sample.MOQ:500.Chlamydia Pneumoniae Antibody IgG Lateral Flow Assay
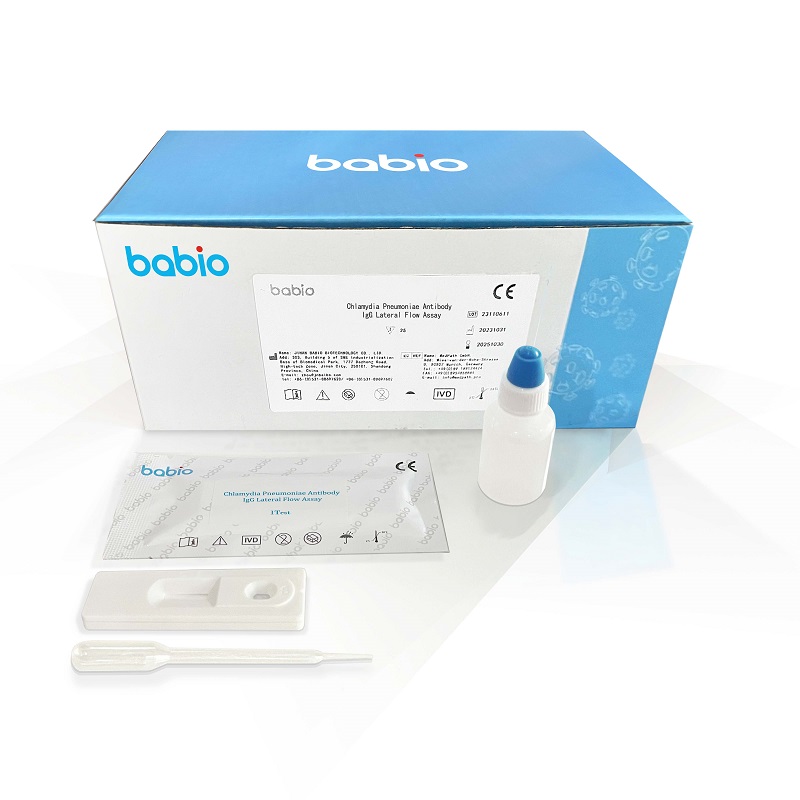
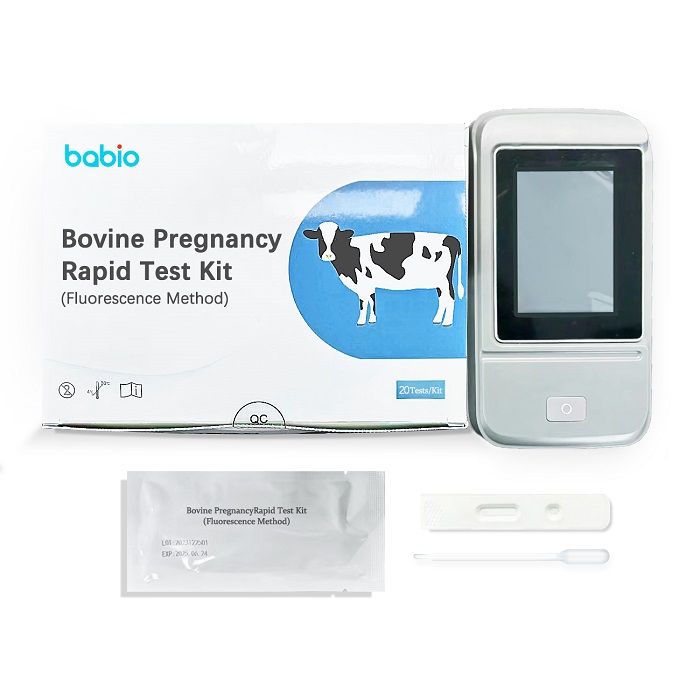
Chlamydia Pneumoniae Antibody IgG Lateral Flow Assay is Chlamydia pneumoniae Antibody IgG gold standard kit (colloidal gold method), for clinical detection of Chlamydia pneumoniae IgG antibody in human serum, plasma or whole blood, for the initial screening or rapid detection of Chlamydia pneumoniae infection.Bovine Pregnancy Rapid Test Kit (Fluorescence Method)
Bovine Pregnancy Rapid Test Kit (Fluorescence Method)R2A Agar Medium
R2A Agar Medium is ideal for microbial monitoring in controlled environments. Perfect for air quality testing, surface microbial detection, and contamination control in pharmaceutical, food, and cosmetic industries. Ensure reliable results with high-pressure sterilization and aseptic packaging.Aflatoxin M1 Rapid Test Cassette
Ensure dairy safety with the Aflatoxin M1 Rapid Test Cassette. This rapid test provides fast and accurate mycotoxin detection in milk and dairy products, meeting EU and FDA safety standards. Trusted by food safety professionals. Learn more at Babio Biotech.JC-1200MB Microplate Reader
The JC-1200MB Microplate Reader by Babio delivers high-precision ELISA plate analysis for clinical diagnostics, microbiology, immunology, and research labs. Featuring a 7-inch LCD touchscreen, multi-wavelength detection, curve-fitting algorithms, and robust software, it ensures accurate and reliable results worldwide.